Cell and Gene Therapy Virus Production
Optimize Recombinant AAV and Lentivirus Production
High yield, high quality, and high-efficiency virus production is essential for advancing the field of cell and gene therapy. Producing enough viral particles to treat a high dose, high prevalence disease poses significant challenges. Manufacturers are forced to scale out and scale-up, driving investment in both CapEx and labor. With Mirus Bio, there’s a better way.
Optimizing your transfection process with the TransIT®-AAViator and TransIT-VirusGEN® Transfection Systems and RevIT AAV Enhancer can yield significant increases in titer while delivering improved virus quality in upstream cell culture. These step-change improvements may eliminate or reduce the need for scaling up and scaling out, achieving significant short term and long-term cost savings.

Mirus Bio
Transfection Reagent + Enhancers
Fewer reactors, less media, less DNA
Fewer production runs, less hands-on time
Efficient use of time and materials, higher profitability
Higher Titers and Percent Full Capsids

VirusGEN plus RevIT AAV enhancer outperformed polymer-only reagents in both titer and percent full capsids
Higher titers lead to significant cost savings and more patient doses
VirusGEN® is the only transfection platform to incorporate proprietary polymer and lipid technology – which enables both high efficiency and productivity.
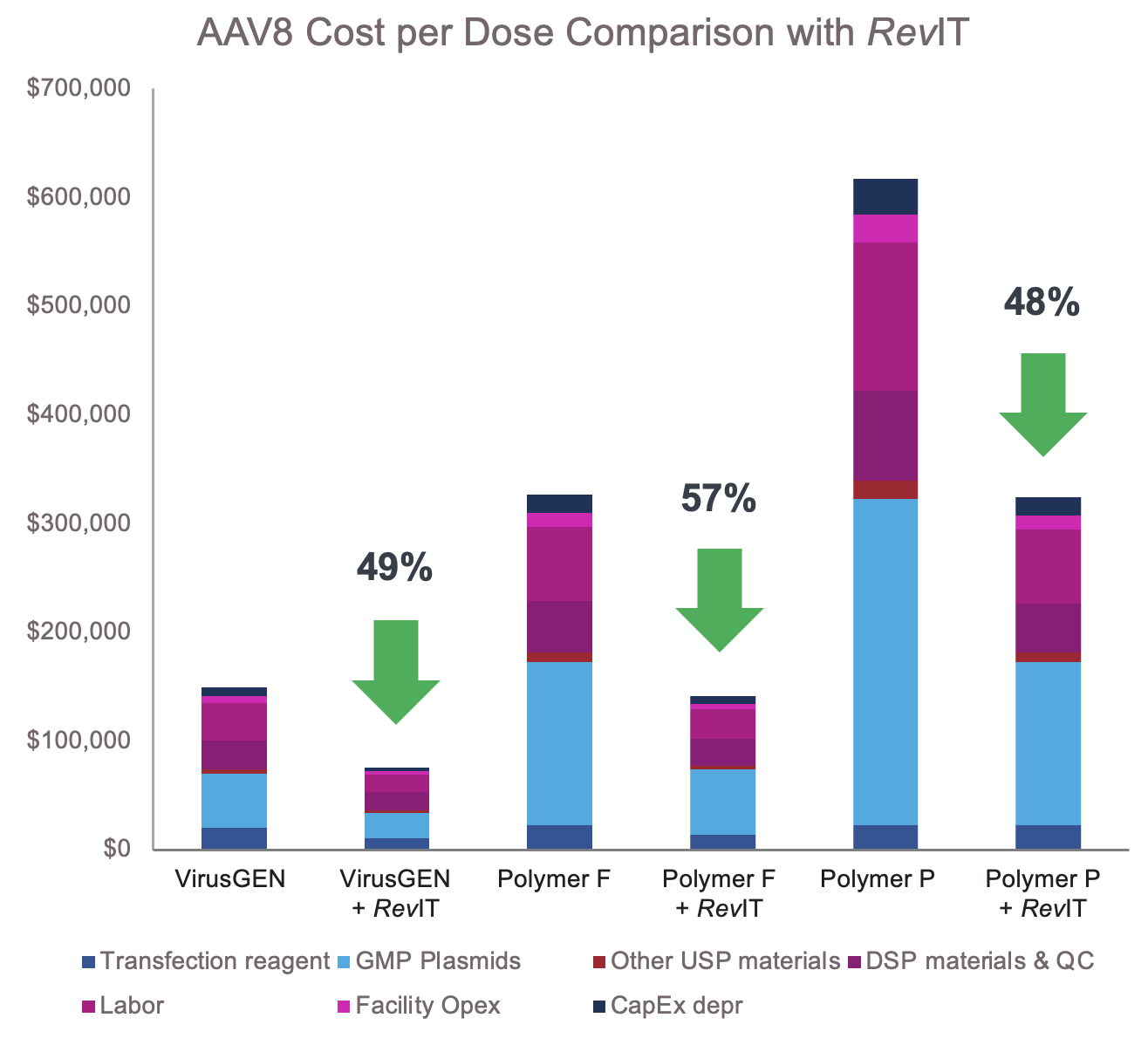
Reduction in cost per dose driven by titer increase with
VirusGEN transfection platform and RevIT AAV enhancer*
Double the number of doses from a 200L bioreactor

*Process conditions: 200L bioreactor run in GMP setting, 3M cells per mL, Reagent : DNA ratios per supplier recommendations RevIT AAV enhancer added post transfection Dose: 5E+14, AAV8
Cell and Gene Therapy Applications
Cell and Gene Therapy Products
TransIT-AAViator Transfection System
VirusGEN Transfection Reagents
-

TransIT-VirusGEN® Transfection Reagent – 0.3 mL
$146.00 Add to cart -

TransIT-VirusGEN® Transfection Reagent – 0.75 mL
$368.00 Add to cart -

TransIT-VirusGEN® Transfection Reagent – 1.5 mL
$583.00 Add to cart -

TransIT-VirusGEN® Transfection Reagent – 10 x 1.5 mL
$4,668.00 Add to cart -

TransIT-VirusGEN® Transfection Reagent – 150 mL
$37,294.00 Add to cart -

TransIT-VirusGEN® Transfection Reagent – 30 mL
$7,980.00 Add to cart -

TransIT-VirusGEN® Transfection Reagent – 5 x 1.5 mL
$2,538.00 Add to cart
RevIT AAV Titer Enhancer
VirusGEN AAV Transfection Kits
-

VirusGEN® AAV Kit + RevIT™ for 1L Culture – With VirusGEN® Stabilizer
$1,162.00 Add to cart -

VirusGEN® AAV Transfection Kit – 1 Kit for 1 L of cell culture
$1,162.00 Add to cart -

VirusGEN® AAV Kit + RevIT™ for 10L Culture – With VirusGEN® Stabilizer
$7,993.00 Add to cart -

VirusGEN® AAV Transfection Kit, 1 Kit – for 10 L of cell culture
$8,202.00 Add to cart -

VirusGEN® AAV Transfection Kit – 150mL
$39,844.00 Add to cart
VirusGEN LV Transfection Kits
GMP Products
ISO 13485:2016 Certified

Completion of this comprehensive certification process underscores the quality of the processes used to support the company’s GMP product portfolio.
View the certificate








